According to research done by www.FDAImports.com, The U.S. Food and Drug Administration (“FDA”) aggressively stepped-up the number of food facility inspections in China last year according to the FDA Inspection Classification Database. Between fiscal year 2009 and 2010, FDA had a thirteen-fold increase in food facility inspections in China. Last year’s dramatic increase foreshadows FDA’s greater international profile through inspecting foreign food facility, which Congress mandated in the recently passed FDA Food Safety Modernization Act (“FSMA”).
China has played an increasingly important role in supplying food to the United States. Between 1999 and 2008, China went from the eleventh largest supplier of U.S. food imports to the third largest. Despite this increased presence, previously FDA only conducted a limited number of food facility inspections in China, as well as all other countries too.
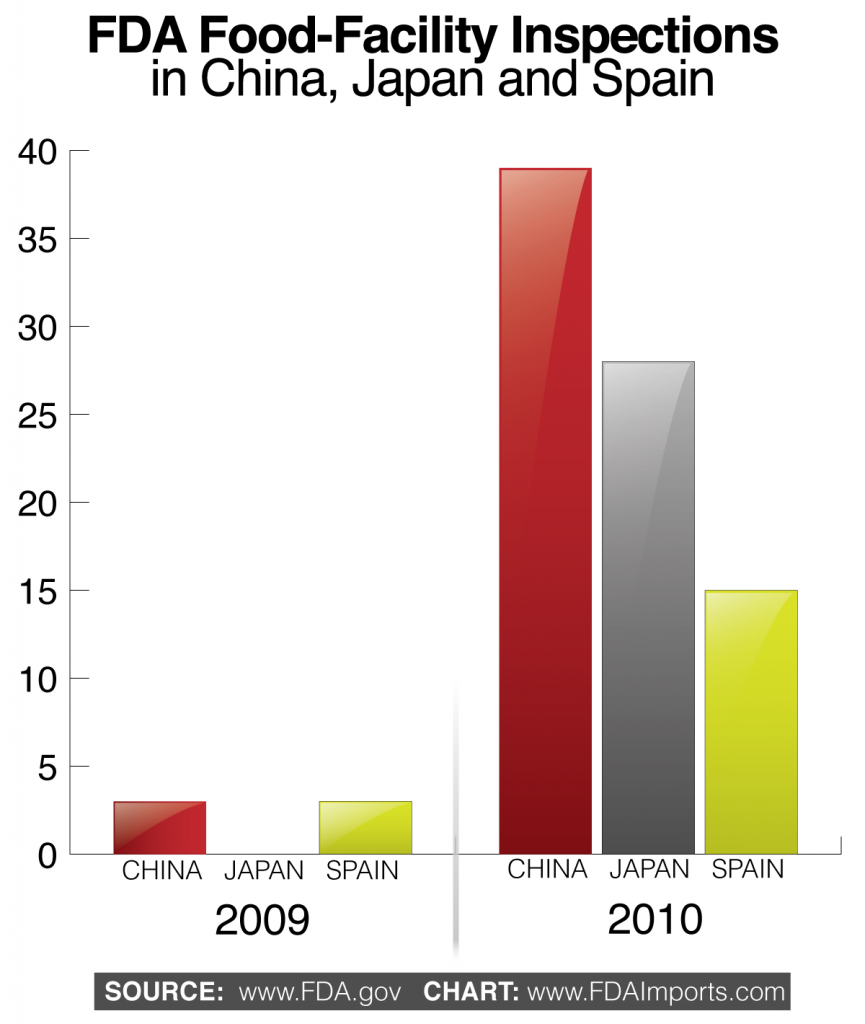
Intense congressional and public scrutiny caused FDA to increase the number of food facility inspections in foreign country. In fiscal year 2009, FDA only conducted three food facility inspections in China. However in 2010, FDA conducted thirty-nine food facility inspections.
FDA’s increased presence in China mirrors its greater activity in other countries. For example, FDA did not conduct any food facility inspections in Japan during fiscal year 2009, but conducted twenty-eight in 2010. In Spain, FDA conducted three food facility inspections in 2009 and fifteen in 2010.
In every food facility inspection in China, FDA inspected for foodborne biological hazards, which included examining for filth, decomposition, and microbiological contamination. FDA primarily inspected seafood and canned-food facilities, which are at higher risk of biological hazards.
Chinese food facilities should become comfortable with FDA’s presence, which will only increase in the coming years. China’s expanding role in the U.S. food supply makes its food facilities highly probable targets for the foreign food facility inspections mandated by FSMA. Seafood and canned-food facilities should remain ever prepared. FDA is likely to continue to focus on these facilities and their regulation compliance, because they present a greater public-health risk as these foods are more probable to become contaminated with biological hazards.
A final note of caution, an FDA food facility inspection is not optional if the facility desires to export its products to the United States. According to FSMA, FDA must refuse entry into the United States of any food from a facility that refused an FDA inspection.
– Benjamin L. England, Founder